Hi everyone I found out that lithium is so soft that it can be cut with a knife!
science e-portfolio
Saturday, September 4, 2010
Saturday, August 28, 2010
Global warming
Hi everyone, today I am going to talk about global warming. Global warming is actually the average temperature of Earth's near-surface air and oceans since the mid 20th century and its projected continuation.
Humans have contributed a lot to global warming. When we burn fossil fuels to generate electricity, a lot of CO2 is being released. These CO2 contribute to global warming by absorbing heat energy from the earth, trapping it and preventing its release into space. When more and more carbon dioxide is produced, more heat energy is trapped in Earth. The increase of carbon dioxide in the atmosphere through fossil fuel consumption and land clearing causes CO2 to form a kind of atmospheric blanket that traps an increasing amount of the earth's heat, causing the global temperature to rise.
Global warming can result to killing every living thing on Earth if it gets too serious, but we can always slow down its rate of destruction to our planet. For example, we can save more electricity, plant more trees, use less vehicles, etc. We can do very simple things such as using energy-saving light bulbs and turning off electrical devices when not in use to save electricity and reduce the amount of fossil fuels needed to be burnt . Many people, including me, are sometimes too lazy to do these little efforts but from now on, all of us should remember that we have to change our bad habits and save our planet from global warming or we will have to suffer the consequences of being extinct. I hope that humans will be willing to change and we will not die due to global warming.

Humans have contributed a lot to global warming. When we burn fossil fuels to generate electricity, a lot of CO2 is being released. These CO2 contribute to global warming by absorbing heat energy from the earth, trapping it and preventing its release into space. When more and more carbon dioxide is produced, more heat energy is trapped in Earth. The increase of carbon dioxide in the atmosphere through fossil fuel consumption and land clearing causes CO2 to form a kind of atmospheric blanket that traps an increasing amount of the earth's heat, causing the global temperature to rise.
Global warming can result to killing every living thing on Earth if it gets too serious, but we can always slow down its rate of destruction to our planet. For example, we can save more electricity, plant more trees, use less vehicles, etc. We can do very simple things such as using energy-saving light bulbs and turning off electrical devices when not in use to save electricity and reduce the amount of fossil fuels needed to be burnt . Many people, including me, are sometimes too lazy to do these little efforts but from now on, all of us should remember that we have to change our bad habits and save our planet from global warming or we will have to suffer the consequences of being extinct. I hope that humans will be willing to change and we will not die due to global warming.

Sunday, August 22, 2010
Why does ice float on water
Hi everyone, now I am going to talk about why does ice float on water.
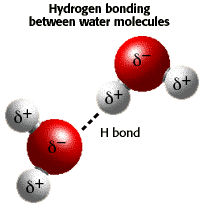
Water reaches its maximum density at 4°C (40°F). As it cools further and freezes into ice, it actually becomes less dense. However, most substances are most dense in their solid state than in their liquid state. Water is different because of hydrogen bonding.
Water reaches its maximum density at 4°C (40°F). As it cools further and freezes into ice, it actually becomes less dense. However, most substances are most dense in their solid state than in their liquid state. Water is different because of hydrogen bonding.
Each water molecule is made of 2 hydrogen atoms and 1 oxygen atom. These are connected to one another by very strong chemical bonds called covalent bonds. Water molecules are connected to each other by much weaker chemical bonds called hydrogen bonds between the positively charged hydrogen atoms, and one negatively charged oxygen atom in a neighboring water molecule.
As water gets colder than 4 degrees Celsius the hydrogen bonds connecting different water molecules adjust to keep the negatively charged oxygen atoms apart. This results in a crystal latice which begins to form at less than 4 degrees Celsius. This crystal latice is completely formed at freezing, and is commonly known as ice.
As water gets colder than 4 degrees Celsius the hydrogen bonds connecting different water molecules adjust to keep the negatively charged oxygen atoms apart. This results in a crystal latice which begins to form at less than 4 degrees Celsius. This crystal latice is completely formed at freezing, and is commonly known as ice.
So, ice floats as it is less dense than water.
Lumminous and non-luminous flames
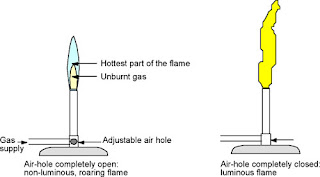
Now I am going to talk about the luminous and the non- luminous flames.
Luminous flame:
-is produced when the air-holes of the bunsen burner are closed and very little air is allowed to mix with the gas
-orange in colour
-appears flickering and unsteady
-not very hot
Non-luminous flame:
-occurs when air-holes of the bunsen burner are open, allowing air into the burner
-blue in colour
-burns steadily
-hotter than the luminous flame
-hottest part of the flame is just above the tip of the blue zone.
Luminous flame:
-is produced when the air-holes of the bunsen burner are closed and very little air is allowed to mix with the gas
-orange in colour
-appears flickering and unsteady
-not very hot
Non-luminous flame:
-occurs when air-holes of the bunsen burner are open, allowing air into the burner
-blue in colour
-burns steadily
-hotter than the luminous flame
-hottest part of the flame is just above the tip of the blue zone.
Saturday, August 21, 2010
Testing if photosynthesis had taken place in the leaf
On 28 July2010, we had an experiment in the lab about finding out if photosynthesys had taken place in the leaf. First, we have to boil the leaf for 1-2 mins to break the cell structure. Then, we stirred the leaf in a test tube of alcohol to remove chlorophyll. After that, we put the leaf on a white tile and dropped iodine on it. The result was supposed to be the iodine turning blue, but my experiment failed and my iodine was still brown. What we could conclude was that photosynthesis had taken place in the leaf.
Reverse osmosis
Today, I am going to talk about reverse osmosis. It is actually a filtration method that removes many types of large molecules and ions from solutions by applying pressure to the solution when it is on one side of a membrane. The solute will then be retained on the pressurized side of the membrane and the pure solvent is allowed to pass to the other side. Here is a diagram of how it works:
Uses of reverse osmosis:
-drinking water purification
-water and wastewater purification
-food industry
-reef aquariums
The membranes used in reverse osmosis have a dense barrier layer in the polymer matrix where most seperation occurs. The membrane is designed to allow only water to pass through this dense layer, and solutes such as salt ions are prevented from passing through. This process requires a high pressure to be exerted on the high concentration side of the membrane, usually 2-17 bar(bar is a unit of pressure roughly equal to the atmospheric pressure on Earth at sea level) for fresh and bracksh water, and 40-70 bar for seawater.
-drinking water purification
-water and wastewater purification
-food industry
-reef aquariums
The membranes used in reverse osmosis have a dense barrier layer in the polymer matrix where most seperation occurs. The membrane is designed to allow only water to pass through this dense layer, and solutes such as salt ions are prevented from passing through. This process requires a high pressure to be exerted on the high concentration side of the membrane, usually 2-17 bar(bar is a unit of pressure roughly equal to the atmospheric pressure on Earth at sea level) for fresh and bracksh water, and 40-70 bar for seawater.
Subscribe to:
Posts (Atom)